INTRODUCTION
Mechanical responsiveness plays an essential role in many biomechanical processes and is common in biological systems [1, 2]. Over the last two decades, significant effort has been made for developing biomimetic mechano-responsive hydrogels capable of altering their physical and chemical characteristics in response to external mechanical stimuli. In this study, a bioinspired hydrogel was developed to replicate tissue-like mechano-responsiveness, exhibiting strain-stiffening and stress-induced differential modulus adaptation. Biomimetic self-assembly endows the material with structural stability, biocompatibility, and enhanced mechanical performance in wound closure applications. Owing to the apparent combination of tissue resemblance and mechano-responsive capabilities, this type of hydrogel offers significant advantages in a wide range of biomedical applications. Based on preclinical research and clinical reports implicating mechanical tension effects, scar formation would be inhibited if mechanical forces were actively offset through stress shielding by activating mechano-responsiveness across the wound [3]. Mechano-responsive materials are also implicated in practically all physiological processes, such as homeostasis and development of organs and tissues [4–7]. It is widely recognized that mechano-responsive materials modulate skin and wound behavior [4, 8–10]. Mechano-responsive materials modulate skin and wound closure through mechanotransduction and extracellular matrix (ECM) remodeling pathways, particularly involving β-integrins. These mechanisms regulate cellular responses to mechanical stimuli, enhancing tissue repair and creating a supportive environment for wound closure. Mechano-responsive hydrogels are categorized into several groups based on properties that change in response to mechanical stimuli, such as viscosity, color, and strength. The most studied response properties in hydrogel systems include strain-stiffening, mechanochromism, self-healing, and shear thinning [1, 11–17]. Strain-stiffening is a mechanical response with a nonlinear force-extension relation. A material can exhibit strain-stiffening when stretched, and a positive strain causes an increase in stiffness along the strain direction [18, 19]. Strain-stiffening hydrogel mimics the physiological features of biological tissues, making them intriguing options for tissue scaffolds, artificial tissues, and wound closure [20–22]. However, if the external stimuli reach sufficiently high levels, it can lead to mechanical failure (fracture) and damage the skin [20]. Strain-stiffening synthetic materials consist of two or more separate networks with varying stiffnesses for synergizing firmness and softness [11, 23].
To the best of our knowledge, only a few studies have shown unique strain-stiffening properties for enhanced wound closure in synthetic materials as most synthetic materials exhibit negligible strain-stiffening properties. Previous research has resulted the development of wound dressings using polyisocyanopeptides that show strain-stiffening properties [21, 22]. The enhancement or acceleration of wound dressing techniques has been the topic of many recent studies. However, studies regarding the use of strain-stiffening properties of mechano-responsive materials for wound-closure applications are very few. Polyampholyte polymers, such as poly (SA-co-TMA), have been widely used as antifouling materials to facilitate wound dressing by enhancing skin re-epithelialization of wounds after injury [24]. Mixed-charge copolymers composed of 3-sulfopropyl methacrylate (SA) and [2-(methacryloyloxy)ethyl] trimethylammonium (TMA) exhibit precise charge balance, high hydration capacity, protein antifouling, and antibacterial activity through their polyampholyte nature. Mechanically, the incorporation with polytetrafluoroethylene (ePTFE) membranes provides high tensile strength, elasticity, fatigue resistance, and dimensional stability, making them suitable for dynamic and load-bearing wound environments.
Herein, we report the design and preparation of a self-assembly (SAM) technique to enhance the mechanical and mechano-responsive properties of TSC polyampholytes. The TSC polyampholyte constitutes a cationic monomer of a 2-(Acryloyloxy ethyl) trimethylammonium chloride (TMA) and two anionic monomers, 3-sulfopropyl methacrylate potassium salt (SA) and 2-carboxyethyl acrylate (CAA), where T, S, and C represents the TMA, SA, and CAA monomers, respectively, and the number represents the molar ratio that includes a cross-linking triethylene glycol dimethacrylate network. The influence of SAM technique on the phase structure, mechanical and mechano-responsive properties was examined. The characteristics of the TSC polyampholytes were studied in detail using IR. An SAM of the TSC polyampholyte hydrogel displayed strain-stiffening properties. In addition, the effects of the TSC polyampholyte hydrogel on mediating the cell fate was studied and optimized. The results indicated that the strain-stiffening property would aim in solving the problem of wound-closure in static or dynamic mechanical environments.
MATERIALS AND METHODS
Preparation of the TSC polyampholyte based on the SAM approach
A typical SAM approach was used for the synthesis of TSC. We synthesized a new type of polyampholyte hydrogel T1SxC1-x by one-step copolymerization of a cationic monomer of TMA and two anionic monomers, SA and CAA. In Milk Q water, 80 μL of a 15% sodium metabisulfite solution and 80 μL of a 40% ammonium persulfate solution were used to begin polymerization, which was slightly different from that used in a previous study [25]. The polymerization procedure was then performed for 1 h at 60°C, after which the gel was cooled for 1 h at room temperature. Following SAM, an 8 M sodium hydroxide (NaOH) aqueous solution was filtered to assemble the TSC building blocks to obtain TSC polyampholyte (SAM).
Sample Characterizations
Infrared spectroscopy (IR) was performed using a Vertex 70 series spectrophotometer (Bruker Optics, USA). This spectrometer was used to study the secondary structure of TSC polyampholyte with different ratios were referred to as T1S0C1, T1C0.5S0.5, and T1S1C0 samples.
Mechanical Characterizations
A tensile machine (FGS-50E-H, Japan) was used to investigate the tensile properties of the TSC polyampholyte. Uniaxial tensile tests were performed in accordance with the ISO 37 Type 3 standard. The strain-stiffening of the material was used to determine the mechanical performance [25]. The differential modulus (k′) of hydrogel was assessed according to the following equation (1) [26].
Here, k′ denotes the differential modulus, defined as a function of stress (σ) and strain (γ), for the same hydrogel.
In vitro Cell culture
The viability of human dermal fibroblasts-adult HDF-a was quantitatively verified by using cell counting kit (CCK-8, Dojindo, Japan) assay on days 3 and 7. The HDF-a cells were seeded in 21 well-cultured plates and then immersed in a CCK-8 reagent-mixed solution at 37°C for 2 h. Finally, viability of HDF-a cell was determined using a plate reader and absorbance was measured at 450 nm. The nuclei of HDF-a cells were stained with 4′,6′-diamidino-2-phenyl-indol (DAPI, Sigma-Aldrich). For cell morphology and viability observation, the samples were stained using fluorescein diacetate (FDA, Sigma-Aldrich) staining assays and photographed after 7 days.
HDF-a cell migration was studied using an in vitro wound closure scratch assay. Firstly, seed the HDF-a cell suspension into each well with the silicone culture insert using 200 μm as a physical barrier. Afterwards, removal of the culture insert will engender a clean cell-free gap. Incubate the dishes for 24 hours at 37°C and 5% CO2 to allow the cells to migrate into [27]. Finally, measurement of cell migration in the central gap area was measured using Image J software.
Statistical analysis
All data were measured in five replicates (n = 5), unless specified otherwise, represented as the mean ± standard deviation. Statistical significance was indicated by (∗) for probability less than 0.05 (P < 0.05), (∗∗) for P < 0.01, (∗∗∗) for P < 0.001, and (∗∗∗∗) for P < 0.0001.
RESULT AND DISCUSSION
Preparation of the TSC polyampholyte based on the SAM approach
In this study, we used copolymerization to prepare TSC polyampholyte hydrogels. The positively charged T (TMA) is repulsive, whereas the negatively charged S (SA) and C (CAA) exert an attractive interaction. TSC polyampholytes were synthesized in three different types with varying numbers of S and C repeat units. In presence of S negatively charged, the –SO3– strong anion (S) was exposed, thus increasing interaction with – COO– weak anion (C) on the TSC networks. After immersion in NaOH solution, negatively charged S and C interact with positively charged T to form structures in which S and C are located at the outer surface under physiological conditions. In presence of NaOH nanoparticles, the –NH3+ positively charged (T) was unexposed, thus preventing interaction with –SO3– negatively charged (S) and –COO– negatively charged (C) on the TSC networks. This process, known as self-assembly (SAM) formation as shown in Fig. 1b, is depicted in Scheme 1a with an illustration. Thus, SEM imaging (Fig. 1) reveals that the self-assembly process promotes the formation of an interwoven fibrous network within the TSC polyampholyte hydrogel matrix, a feature that is lacking in the non-assembly control samples. These nanofibrillar structures result from the spontaneous organization of triblock copolymers into β-sheet-like domains, as further supported by FTIR analysis (Fig. 3).
Scheme 1.
Schematic Diagram Illustration (a) Preparation of TSC Polyampholyte hydrogel (b) Preparation of TSC-SS Polyampholyte under mechanical force (uniaxial stress)
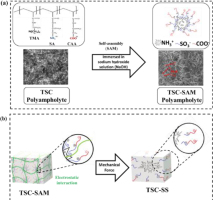
Fig. 1.
Scanning electron microscopy (SEM) images of TSC polyampholyte hydrogel (a) before and (b) after self-assembly process

The C and S functional groups were protonated after immersion in aqueous 8 M NaOH. Mechanical force can weaken the electrostatic interaction between C and S, resulting in detachment from the complex in Scheme 1b.
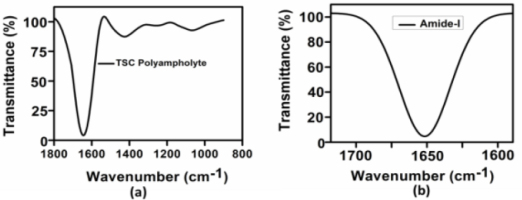
The IR analysis results show that the absorption bands of the hydrogels are characteristic amide-I bands as shown in Fig. 2a–b below [28, 29].
Fig. 2.
Characterization of TSC polyampholyte (a) IR spectra of the TSC Polyampholyte the representative of the IR absorbance spectra of the TSC Polyampholyte (b) The amide-I band region (~1600-1700 cm−1)
The IR spectra in the amide-I band of TSC polyampholyte as shown in Fig. 3 with different ratios (Tab. 1). The amide-I induced pathogenic amyloid transitions that play an important role in immunological homeostasis during wound healing [30].
Fig. 3.
Spectra of protein secondary structure analysis (a) the IR absorbance spectra's second derivatives contained to the amide-I band (b) IR spectra in the amide-I were fitted by approximating the number and position, which experimental curve (▪▪▪) and simulated fits (---) (c) Result simulated fits are the six Gaussian band prof.
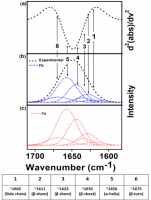
Tab. 1.
Amide-I number and position (cm−1) of TSC polyampholyte
Preparation of the TSC polyampholyte based on the SAM approach
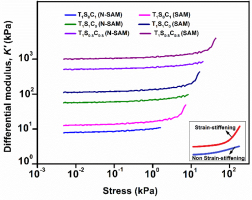
A uniaxial tensile test was performed to validate the increase in the mechanical performance of the TSC polyampholytes. The TSC polyampholytes (T1SxC1-x) with different molar concentrations are referred to as T1S1C0, T1S0.5C0.5, and T1S0C1. In this study, a variety of TSC polyampholytes were manufactured with different SC molar concentrations, using both self-assembly (SAM) and non-self-assembly (N-SAM) techniques. The TSC (SAM) with the SAM technique exhibits strain-stiffening as opposed to TSC (N-SAM) with no self-assembly. T1S0.5C0.5 (SAM) exhibits an optimal differential modulus as shown in Fig. 4. The experimental results thus suggest that strain-stiffening properties enhance the mechanical performance and mechano-responsiveness of TSC polyampholyte.
Strain-stiffening-mediated cell fate
Considering the results of the strain-stiffening analysis, it is possible to hypothesize that during tensile loading, cell bodies are pushed against the surroundings and contact each other, resulting in tensile resistance and strain-stiffening [25]. The current study reveals that the strain-stiffening property of tension may be considered to mediate cell fate. To evaluate the effect of strain-stiffening on the mediated cell fates, human dermal fibroblasts-adult (HDF-a) were cultured under controlled conditions, and no strain-stiffening (TSC-Non-SS) and strain-stiffening (TSC-SS) were calculated as Fig. 5. After 7 days of cultivation, HDF-a cells adhered well to the surface of the matrix. Strain-stiffening also guides the cell-cell-sensing process of cell adhesion [31]. Adhesion of cells (proliferation and differentiation) enhances with the strain-stiffening of TSC-SS as Fig. 5.
Fig. 5.
The cell viability of HDF-a of the different culture plate (control), TSC-Non-SS polyampholyte and TSC-SS polyampholyte
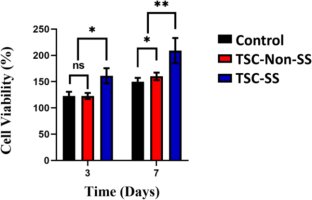
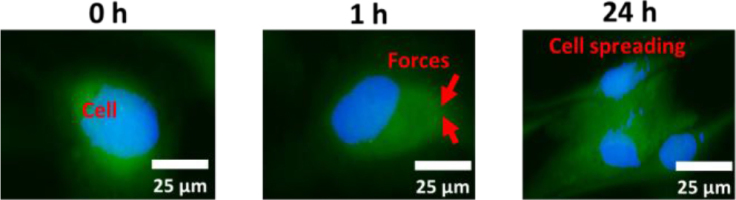
One interesting outcome was the effect of the TSC polyampholyte on the increase in HDF-a content. This effect can be explained by the strain-stiffening property of the TSC polyampholyte hydrogel. Strain-stiffening mediates cell spreading on the hydrogel matrix. Cell spreading of HDF-a cells enhances with the strain-stiffening of TSC polyampholyte (TSC-SS) as Fig. 6.
The results demonstrate that under tensile loading, cell bodies are compressed against their surrounding microenvironment, promoting cell-cell contact. This interaction enhances tensile resistance and increases cell stiffness. The resulting increase in stiffness induces strain-stiffening, a mechanical response that appears to critically influence cell fate determination.
The strain-stiffening TSC could effectively mediate the cell migration than TSC-Non-SS and cultured plates after 24 hours treatment as Fig. 7.
Fig. 7.
In vitro cell culture, strain-stiffening polyampholyte (TSC-SS), nonstrain-stiffening polyampholyte (TSC-Non-SS) and control induce motility in HDF-a cells after 1 day. Scale bar = 200 μm
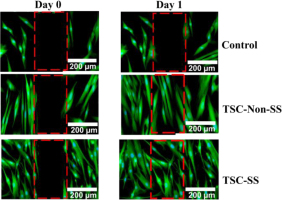
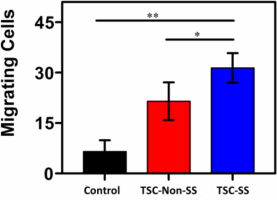
TSC-SS demonstrated higher migrating activity, which could be represented as number of cells filling the central gap as Fig. 8. Furthermore, the strain-stiffening TSC could effectively mediate the cell fate of HDF-a to promote a general organizing principle during tissue development or regeneration. Overall, TSC-SS polyampholytes are promising for wound closure applications.
CONCLUSIONS
In this study, we developed mechano-responsive materials and a self-assembly (SAM) technique to evaluate the strain-stiffening property of TSC polyampholytes for wound closure applications. The TSC hydrogel has amide content, and its differential modulus, contributed to the excellent strain-stiffening property of the TSC polyampholyte. Therefore, strain-stiffening also guides mediated cell fate and is used to connect cells. In addition, the cell migration results indicated that the TSC-SS hydrogel took a shorter time to heal the wound than the TSC-non-SS hydrogel and significantly accelerated the wound closure process with less wound closure time. The strain-stiffening property is a damper response to external mechanical forces and promotes wound closure. Such tough strain-stiffening hydrogels hold great promise for treating wounds in static or dynamic mechanical environments.